 Early February the European Commission published the “Delegated Acts” on safety features in the Official Journal of the European Union, supplementing Directive 2001/83/EC (Falsified Medicines Directive). This publication effectively marks the start of a three year implementation period for all EU member states.
Early February the European Commission published the “Delegated Acts” on safety features in the Official Journal of the European Union, supplementing Directive 2001/83/EC (Falsified Medicines Directive). This publication effectively marks the start of a three year implementation period for all EU member states.
The deadline for implementation is 9 February 2019.
Not all Pharmaceutical Manufacturer had been to follow the going alive of the so called Delegated Act closely. However, some did because they have been warned to implement Serialisation into their production due to other national demands seen in markets such as Turkey, China or Korea. Soon one can only sell prescription drugs when produced and marked in compliance with Directive 2001/83/EC. Furthermore, the US Government is expected to announce their demands and regulations for the Traceability of medicine in the North America.
The following provides a good example on full application line installed at Aenova. A story about Discovering Simplicity by using the OCS Track and Trace solution “TQS”.
The Aenova Group is one of the world’s leading pharmaceutical and healthcare companies. They employs more than 4,000 employees at 20 locations. As the European market leader in the business-to-business area, the group relies on high quality standards, innovative technologies, and a clear focus on the future. These are the reasons why, with the coming serialisation requirement for drugs, it has chosen to trust in the proven success of “Traceable Quality System” (TQS), the Track & Trace solution by OCS Checkweighers.
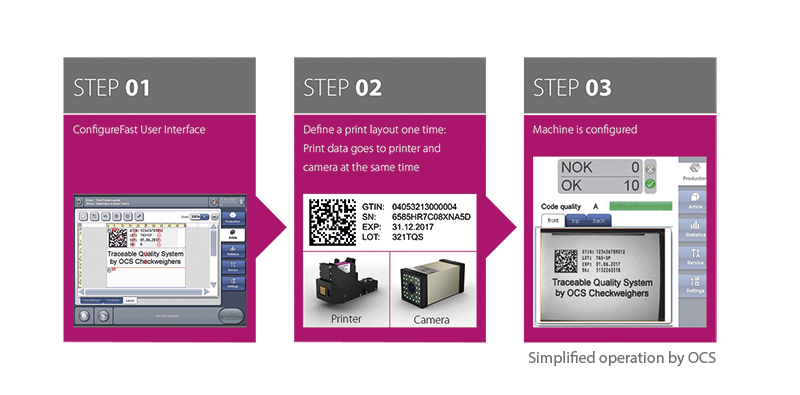
Alexander Pohl is Manager of the corporate packaging department and remembers his first contact and the start of the good support from the OCS sales team. In addition to the very good service offer, it was the competent, open, and transparent dialog that contributed from the start to giving OCS a major advantage over the competition. “Ultimately, it was the innovative operating concept,” said Alexander Pohl, “which is really simple and easy to learn.” All system components of TQS can be controlled directly from one, convenient software interface without switching programs. “That is what made it the clear winning bid,” remembered Pohl.
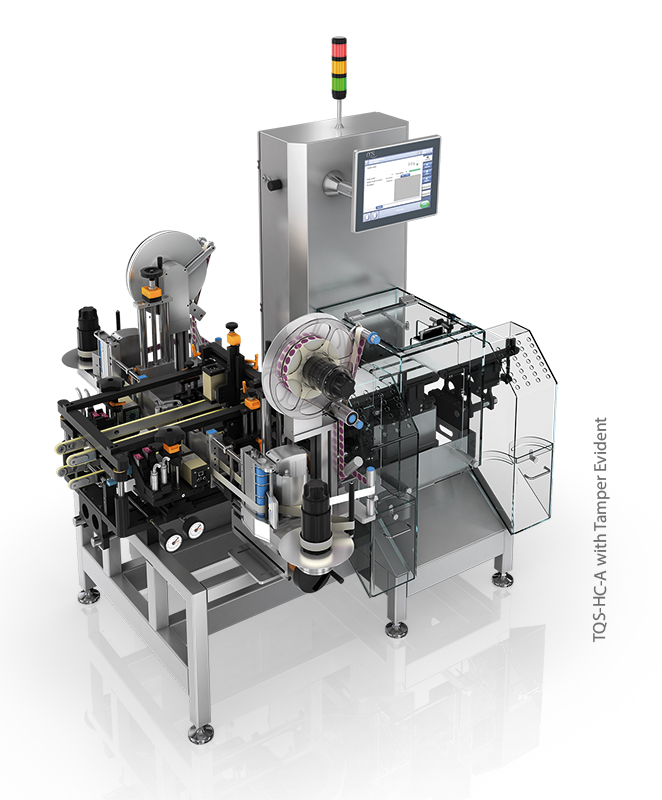 An anti-inflammatory drug moves along on the conveyors and must be provided with a special code for export to China. The build-in high precision product transport is absolutely necessary for 1D coding (which is difficult in any case) at transport speeds of up to 60 m/min. First class grading (minimum “B”) with subsequent verification can now be achieved. If there are no complaints about the coding from the camera control, the product moves on to the highly accurate weight control, which confirms the completeness of each individual folded box. Packages that have a coding error or an incorrect weight are independently separated and diverted into lockable collection containers.
An anti-inflammatory drug moves along on the conveyors and must be provided with a special code for export to China. The build-in high precision product transport is absolutely necessary for 1D coding (which is difficult in any case) at transport speeds of up to 60 m/min. First class grading (minimum “B”) with subsequent verification can now be achieved. If there are no complaints about the coding from the camera control, the product moves on to the highly accurate weight control, which confirms the completeness of each individual folded box. Packages that have a coding error or an incorrect weight are independently separated and diverted into lockable collection containers.
Both processes – serialisation and completeness check – are performed on the TQS-HC-A, a component of the Traceable Quality System, which combines perfect product labelling with the high precision and globally respected weighing technology.
The Weigh Cells used in the TQS operate on the principle of electro-magnetic force restoration (EMFR). This process provides significantly faster settling times and, consequently, faster measurement results than comparable methods of dynamic weighing on an industrial scale.
At Aenova, approximately 300 folding boxes per minute are reliably checked for exact weights (OCS weighing technology is capable of weighing 640 pieces / minute legal for trade compliant).
After the cartons have successfully passed the TQS-HC-A, they are packed by stretch banding into bundles with 10 cartons per bundle. Subsequently, these bundles are forwarded to the next Track & Trace component TQS-BP (Bundle Pack) for implementation of the first level of aggregation. This is where the serial numbers of all ten individual cartons are summarised to create a separate and unique serial number for the bundle. This label is applied to the bundle (see left photo), before completing the final steps of multi-code reading of the serial numbered individual packages and then the verification of the bundle label.
The integrated system for complete aggregation
 The individual bundles eventually reach the TQS-CP, the case packing component of the Track & Trace integrated solution from OCS Checkweighers. The TQS-CP handles the aggregation in the area of manual packaging. This is where the separate bundles are initially packed layer by layer into a shipping carton. After each layer is completed, the fully integrated camera takes a high resolution photo (16 megapixel) of the full surface area showing all of the individual codes in that layer. After all layers of the shipping carton have been processed, the aggregation is closed out and a label is automatically generated for the shipping carton.
The individual bundles eventually reach the TQS-CP, the case packing component of the Track & Trace integrated solution from OCS Checkweighers. The TQS-CP handles the aggregation in the area of manual packaging. This is where the separate bundles are initially packed layer by layer into a shipping carton. After each layer is completed, the fully integrated camera takes a high resolution photo (16 megapixel) of the full surface area showing all of the individual codes in that layer. After all layers of the shipping carton have been processed, the aggregation is closed out and a label is automatically generated for the shipping carton.
Furthermore, each separate TQS component on the production line is networked by the Line Manager.
Alexander Pohl is excited about the successful installation of the Track & Trace integrated solution from OCS Checkweighers, which he described as a flexible, dynamic, and innovative company. “We feel that we are in good hands with OCS. With regard to the mandated serialisation it is the best solution we could find.”

OCS Checkweighers Ltd.
Tel: +44 1993 701-970

